
当前位置:首页 > 企业查询 > 上海 > 上海双墨生物科技有限公司 > 产品服务 >
KinExA完整细胞亲和力测定仪 相互作用




KinExA?分子与细胞相互作用系统介绍
一、KinExA的?技术背景:
Sapidyne Instruments Inc.于1995年在美国创立,产品基于独特的Kinetic Exclusion Assay(KinExA)专利技术。在公司成立早期,Xavier大学、美国陆军和环境保护局等研究单位采用KinExA技术开展了大量工作;经过数十年在生物制药领域、科研领域及环境监测领域的广泛应用,KinExA技术已成为*制药公司和生物技术公司以及许多大学、独立研究实验室和环境监测机构研究相互作用和生物活性物质检测的必备工具,并且已得到FDA和EMA认可。
二、KinExA的技术原理:
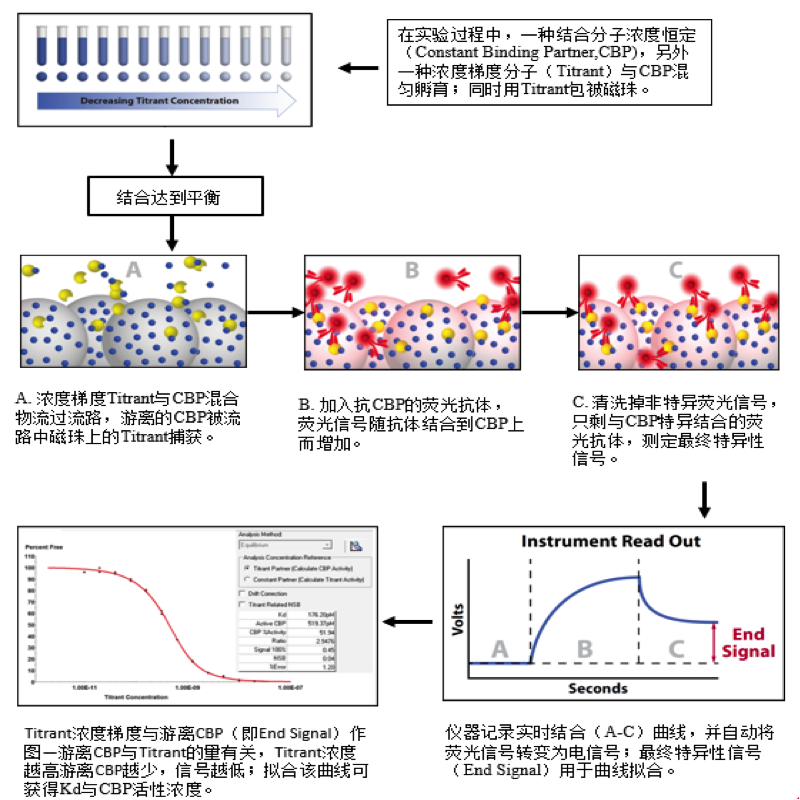
在反应溶液中当受体和配体达到平衡状态时,这时溶液中存在三种物质:受体、配体以及受体-配体复合物。KinExA技术通过包被受体或配体的珠子在极短时间内(<0.5s,不影响反应平衡)捕获游离的配体或受体,再通过荧光标记的抗体检测游离的配体或受体的量。
三、KinExA与SPR的区别
1、与SPR的区别:SPR在芯片表面固定一个分子,通过芯片表明与溶液间二维相互作用的物质量改变而实现SPR检测。这就带来了非常显著的缺点:固定在芯片上的生物分子可能不能维持其天然活性、质量迁移影响动力学分析(例如,流速会影响实验结果)、被检测分子有分子量下限限制、非常大的分子或者生物结构其分子量有上限限制、样品需要纯化及无法检测完整细胞。相反,KinExA分析三维水平及游离状态相互作用,不固定任何分子、不会对平衡带来影响、没有质量迁移的限制、可以检测未纯化样品和完整细胞;因此,极宽范围内的生物分子、生物结构及完整细胞均可灵活分析。
2、与SPR技术对比:为了表征治疗性单克隆抗体候选分子,研究者采用不同类型芯片,从Biacore系统获得同一组单抗-抗原的53组数据,与KinExA实验数据对比发现,亲和力及动力学数据与所使用的芯片类型有关,带负电荷的CM5,CM4及CM1芯片对Biacore的动力学数据有不利的影响。为了验证这一假设,作者通过Biacore液相实验,KinExA平衡态滴定以及KinExA动力学实验,精确计算抗体与抗原的亲和力及动力学参数。结果表明随着芯片表面负电荷的降低,亲和力及动力学参数与液相实验所得的结果越接近。可能的原因:(1)带负电荷的葡聚糖芯片与抗体之间的空间位阻影响抗原的结合;(2)带负电荷的抗原与芯片表面的负电荷静电排斥。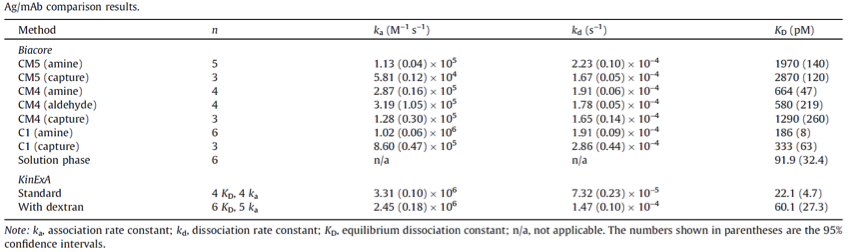
四、KinExA的应用:
应用一、KinExA在CAR-T细胞治疗中的应用
因多种因素的限制,自体CAR-T细胞治疗产品可能在应用于患者前未必能够进行全部项目的检定,所以工艺验证非常重要。工艺研究及验证必须结合工艺的实际情况设定相应的验证参数,CAR-T细胞产品工艺研究在不断发展,到目前为止,业界对何种工艺*好并未达成共识。因此,可以在产品研发过程及早期临床试验阶段开展不同程度的工艺验证研究,工艺验证完成后,应在关键工艺步骤设置关键过程控制参数及标准,以提高CAR-T细胞产品的生产一致性。
CAR-T细胞治疗产品的质量控制研究及检测项目一般应包括:细胞数量及其存活率、细胞表型、CAR阳性率检测、生物学效力检测,无菌检测、支原体、热原/内毒素的检测、CAR-T细胞中病毒载体拷贝数及整合的检测等。如果能够检测CAR-T细胞与抗原分子的亲和力,以及CAR-T细胞上抗体的表达量,无疑会提升CAR-T细胞的质量控制标准。
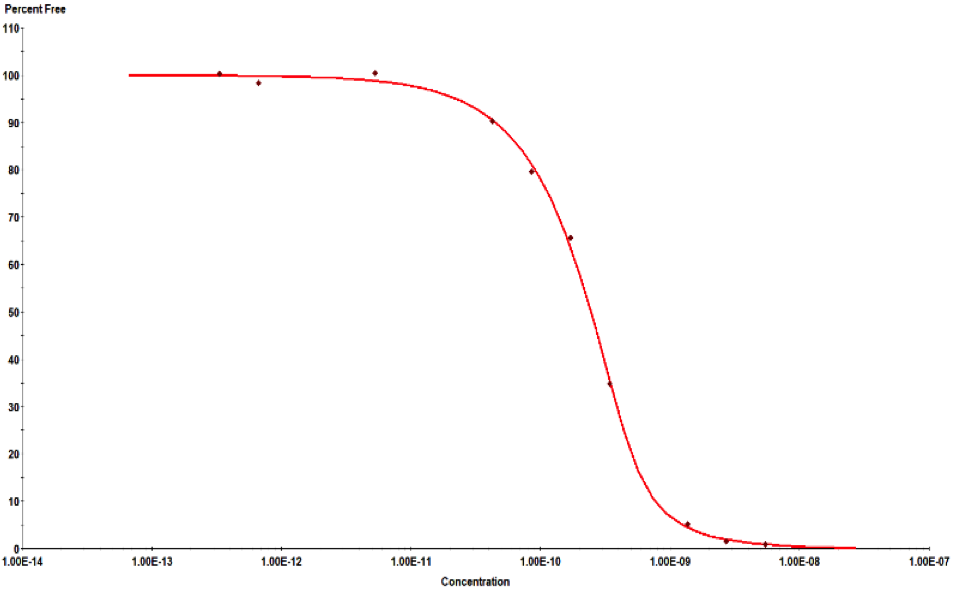
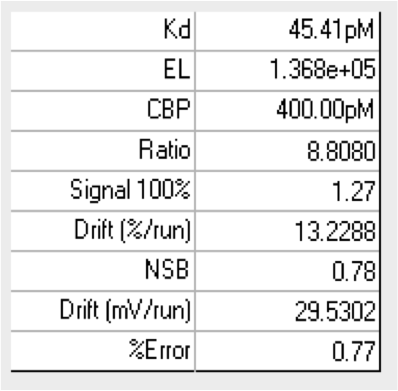
目前市面上除了KinExA技术外,没有其他特别有效的方式检测完整细胞与分子间的亲和力,更无从判断细胞上分子的表达量。KinExA技术可以检测细胞与分子间的亲和力,并且可以计算细胞上分子的表达量。其检测原理如下图,先将梯度稀释的细胞与恒定浓度的分子共同孵育达到平衡,离心之后收集上清液(此时上清液中只存在游离的分子),再通过已提前包被过的珠子捕获游离的分子,用荧光标签的抗体检测游离分子的量,通过检测器检测得到相应的数值后,利用KinExA系统软件进行数据分析。
下图是国内某CAR-T企业使用KinExA系统测定抗原与CAR-T细胞的亲和力,以及T细胞上CAR表达水平的结果:
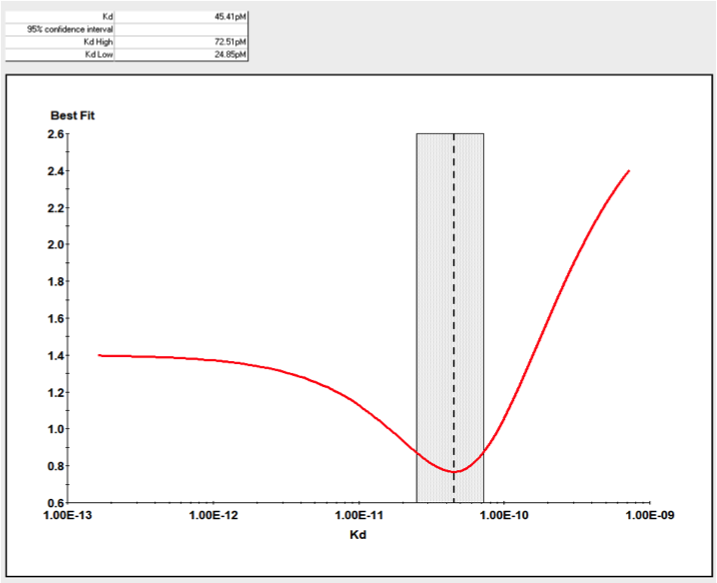
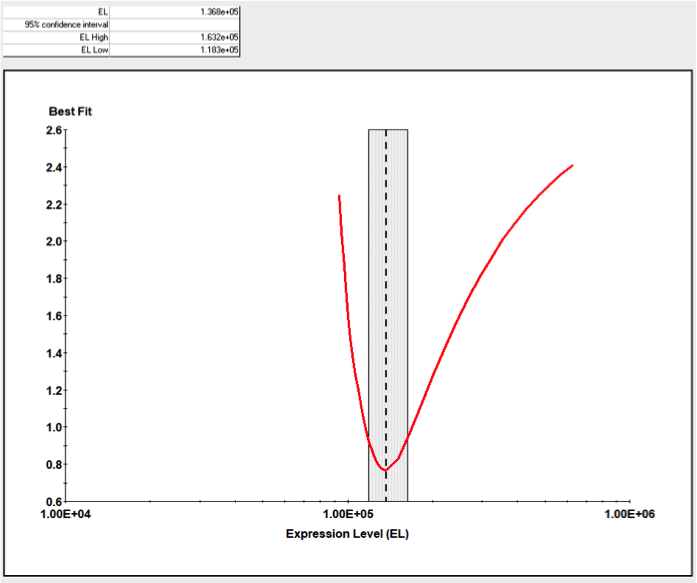
应用二、KinExA在高亲和力检测上应用
对于抗肿瘤药市场,目前精准医疗*为成熟的领域还是以靶向药物为代表的抗肿瘤药物。由于单克隆抗体类抗癌药的副作用较小,且靶向性更好,因此,单抗药物仍将是引领抗肿瘤药物发展*为重要的领域。以PD-1为靶点的一类单抗药呈现出较高的亲和力,常规的相互作用检测系统例如SPR 、BLI、ITC等由于自身原理的限制均不能检测高亲和力(pM级别)。KinExA技术有别于常规的相互作用检测系统,能准确有效的检测高亲和力(fM级别)。
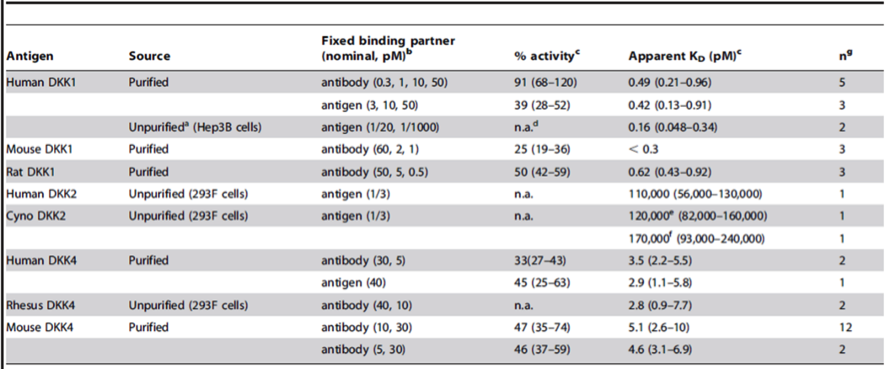
六、KinExA参考文献
完整细胞检测:
? Bedinger, D., et al. 2015. Differential pathway coupling of activated insulin receptor drives signaling selectivity by XmetA, an allosteric partial agonist antibody. J Pharmacol Exp Ther 353(1):35-43.
? Fen Lin,Robin Elliott., et al. 2013.Generation and Characterization of Fully Human Monoclonl Antibodies Against Human Orai1 for Autoimmune Disease. J Pharmacol Exp Ther 345:225-238.
? Rathanaswami P., Babcook J., Gallo M. 2008. High-affinity binding measurements of antibodies to cell-surface-expressed antigens. Anal Biochem 373: 52-60.
? Xie L., et al. 2005. Measurement of the functional affinity constant of a monoclonal antibody for cell surface receptors using kinetic exclusion fluorescence immunoassay. J Immunol Methods 304: 1-14.
未纯化抗原检测:
? Wani T.A., et al. 2016. Analytical Application of Flow Immunosensor in Detection of Thyroxine and Triiodothyronine in Serum. Assay Drug Dev Technol.14(9):535-542.
? Bee C., et al. 2013. Determining the binding affinity of therapeutic monoclonal antibodies towards their native unpurified antigens in human serum. PLOS ONE 8(11): e80501.
? Fujino, Y., et al. 2012. Robust in vitro affinity maturation strategy based on interface-focuses high-throughput mutational scanning. Biochem Biophys Res Commun 4283:395-400.
? Rathanaswami P., et al. 2011. Kinetic analysis of unpurified native antigens available in very low quantities and concentrations. Anal Biochem 414: 7-13.
高亲和力检测:
? Abdiche YN. et al. 2016. Assessing kinetic and epitopic diversity across orthogonal monoclonal antibody generation platforms. MAbs. 10.1080/19420862.2015.1118596.
? Owyang A.M., et al. 2011. XOMA 052, a potent, high-affinity monoclonal antibody for the treatment of IL-1B-mediated diseases. mAbs 3(1): 49-60.
? Champagne K., Shishido A., Root M.J. 2009. Interaction of HIV-1 inhibitory peptide T20 with gp41 N-HR coiled coil. J Biol Chem 284: 3619-3627.
? Kostenuik P.J., et al. 2009. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL J Bone Miner Res 24: 182-195.
? Luginbuhl B., et al. 2006. Directed evolution of an anti-prion protein scFv fragment to an affinity of 1 pM and its structural interpretation. J Mol Biol 363: 75-97.
? Rathanaswami P., et al. 2005. Demonstration of an in vivo generated sub-picomolar affinity fully human monoclonal antibody to interleukin-8. Biochem Biophys Res Comm 334: 1004-1013.
与SPR比较:
? Fleming JK, Wojciak JM. 2017. Measuring Sphingosine-1-Phosphate: Protein Interactions with the Kinetic Exclusion Assay. Methods Mol Biol. 10.1007/7651_2017_5.
? Abdiche YN. et al. 2016. Assessing kinetic and epitopic diversity across orthogonal monoclonal antibody generation platforms. MAbs. 10.1080/19420862.2015.1118596.
? Kusano-Arai 0., et al. 2016. Kinetic exclusion assay of monoclonal antibody affinity to the membrane protein Roundabout 1 displayed on baculovirus. Anal Biochem. 10.1016/j.ab.2016.04.004.
? Drake A.W., et al. 2012. Biacore surface matrix effects on the binding kinetics and affinity of an antigen/antibody complex. Anal Biochem. 429(1):58-69.
KinExA在药物研发中的应用
? Danial M, et al. 2017.Site-Specific Polymer Attachment to HR2 Peptide Fusion Inhibitors against HIV-1 Decreases Binding Association Rates and Dissociation Rates Rather Than Binding Affinity. Bioconjug Chem. 10.1021/acs.bioconjchem.6b00540.
? Fleming JK, Wojciak JM. 2017. Measuring Sphingosine-1-Phosphate: Protein Interactions with the Kinetic Exclusion Assay. Methods Mol Biol.10.1007/7651_2017_5.
? Bedinger, D., et al. 2015. Differential pathway coupling of activated insulin receptor drives signaling selectivity by XmetA, an allosteric partial agonist antibody. J Pharmacol Exp Ther 353(1):35-43.
? Kariolis MS, et al. 2017. Inhibition of the GAS6/AXL pathway augments the efficacy of chemotherapies. J Clin Invest. 10.1172/JCI85610.
? Fan Y., et al. 2016. Immunological Characterization and Neutralizing Ability of Monoclonal Antibodies Directed Against Botulinum Neurotoxin Type H. The Journal of Infectious Diseases 15;213(10):1606-14.
? K?ck, K., et al. 2015. Preclinical development of AMG 139, a human antibody specifically targeting IL-23. British Journal of Pharmacology 172:159-172.
? Tabrizi M.A., et al. 2009. Translational strategies for development of monoclonal antibodies from discovery to the clinic. Drug Discov Today 14(5/6): 298-305.
免疫检测技术:
? Darwish I.A., et al. 2013. Kinetic-exclusion analysis-based immunosensors versus enzyme-linked immunosorbent assays for measurement of cancer markers in biological specimens. Talanta 111: 13-19.
? Prieto-Simon B., Miyachi H., Karube I., Saiki H. 2010. High-sensitive flow-based kinetic exclusion assay for okadaic acid assessment in shellfish samples. Biosens Bioelectron 25: 1395-1401.
? Sasaki K., Oguma S., Namiki Y., Ohmura N. 2009. Monoclonal antibody to trivalent chromium chelate complex and its application to measurement of the total chromium concentration. Anal Chem 81: 4005-4009.
? Glass T.R., Ohmura N., Saiki H. 2007. Least detectable concentration and dynamic range of three immunoassay systems using the same antibody. Anal Chem 79: 1954-1960.
? Bromage E.S., et al. 2007. The development of a real-time biosensor for the detection of trace levels of trinitrotoluene (TNT) in aquatic environments. Biosens Bioelectron 22: 2532-2538.
? Sasaki K., Glass T.R., Ohmura N. 2005. Validation of accuracy of enzyme-linked immunosorbent assay in hybridoma screening and proposal of an improved screening method. Anal Chem 77: 1933-1939.
? Glass T.R., et al. 2004. Use of excess solid-phase capacity in immunoassays: advantages for semicontinuous, near-real-time measurements and for analysis of matrix effects. Anal Chem 76: 767-772.
? Ohmura N., Lackie S., Saiki H. 2001. An immunoassay for small analytes with theoretical detection limits. Anal Chem 73: 3392-3399.
































